Contact Terry Maguire by email for Smoking Cessation Help and Advice. Smoking The dangers of smoking have been known since the 1960s, however the long-term risk was only fully quantified in the 1990s with the publication of a 40 year cohort study of British doctors. Comparing overall survival of smokers with non-smokers between 1951 and 1991, this study found that in those aged 39 to 69 years (“middle-age”), 41% of smokers died compared to 20% of non-smokers. In those who smoked 25 or more cigarettes daily, 50% died in middle-age. It has been estimated that in 2000 there were 114,000 UK deaths attributable to smoking that equates to 22% of all male and 16% of all female deaths. Smoking is an important cause of cancers of the lung, larynx, pharynx, oesophagus, bladder, kidney and pancreas. Overall, around 1/3rd of cancer deaths in men (36%) and 1/5th in women (21%) are attributable to smoking. Smoking is an important cause of cardiovascular disease. The British doctors’ cohort study found that mortality from coronary heart disease was 50% higher in smokers (over 75% in heavy smokers) than in non-smokers. Overall, over 1/8th of cardiovascular deaths (14% in men;12% in women) are attributable to smoking. Smoking is the main cause of chronic obstructive lung disease (COPD) and a cause of pneumonia and also causes or aggravates a wide variety of non-fatal illnesses including asthma, osteoporosis, peptic ulcer, erectile dysfunction, chronic rhinitis and multiple sclerosis. Stopping smoking has major health benefits. Smokers who quit before the age of about 35 years have a life expectancy only slightly less than a non-smoker and quitting at any age provides both immediate and long-term health benefits. Smoking Cessation ServicesSmoking cessation services have been shown to be successful. Currently 1 in 7 (14.6%) of users of the English Smoking Treatment Service successfully stopped smoking and were still not smoking after 52 weeks. The study noted that older users, those who smoked for pleasure (rather than to cope) and those who were extremely determined were more likely to quit whereas those from low socio-economic groups, who smoked their first cigarette of the day within 5 minutes of waking or had another smoker in the house were less likely to succeed. Similar success rates have been demonstrated in pharmacy based smoking cessation services with 14.3% of smokers who used a combination of pharmacist support plus NRT still not smoking after 52 weeks. This compared to 2.7% in a control group that did not receive behavioural support but did use NRT. This shows the impact of behavioural support in smoking cessation initiatives – the more intense the behavioural support the greater the cessation rates at 52 weeks. Table 2
Stopping smoking and pharmacy Most pharmacists agree they have a role in smoking cessation but can identify a number of barriers to doing more in this important area of public health. Barriers identified included; money and time to provide a service, the lack of know-how or training. Space may not be readily available within the pharmacy for one-to-one consultation. Yet there are considerable benefits from providing smoking cessation services to the pharmacy business and to the profession. Tackling the barriersThe pharmacy smoking cessation services now provided across the UK are designed to minimise barriers to involvement. Pharmacies can create profitable income streams from smoking cessation through service payments and sales of NRT products. Training is available from many organisations. Yet many pharmacists still view smoking cessation with ambivalence. Few pharmacists would suggest smoking cessation is not a role for pharmacy but many fail to take a more active role. In light of what we now know about smoking and its effects on health, it could be viewed as unethical to ignore smoking status during a professional encounter. Smokers’ opinions of stopping. A survey by the Health Education Authority (1996) gave an important insight to smokers needs when stopping. Smokers believed willpower was key to success and that most products and services do not impact on personal willpower. Interestingly the survey identified a stigma associated with seeking help to quit smoking. Smokers didn’t like help from “medical” personnel as they perceived them as “bullying”. This point has been endorsed recently in a consumers’ survey which showed that 33% of smokers worried about asking their GP for NRT whereas in the same survey 88% said that they would be comfortable approaching their pharmacist for advice. In the HEA survey 50% of the smokers were reluctant to use NRT as they thought it caused cancer. A more recent (2005) survey has shown that public concerns about the safety of NRT remains a major barrier to the use of NRT products. StoppingStopping, for many smokers, is difficult. To succeed, they require education, support and motivation. Smoking is addictive in three ways;
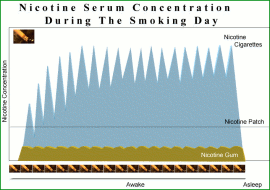
Willpower alone is not sufficient for the vast majority of smokers. Behaviour support plus use of NRT or buproprion have been shown to significantly improve the chances of success. NRT Formulations Six formulations of NRT are currently licensed in the UK: chewing gum, transdermal patch, inhalator, lozenges, sublingual tablet and nasal spray. All are P or GSL medicines. Some were switched to GSL in recent years to improve availability but there is little evidence that this improves cessation rates and it seems that the intervention of a healthcare professional, providing support and encouragement, is important. All NRT formulations are available on Health Service prescription which allows their inclusion in PDGs – a tool by which pharmacies can be paid for supply of NRT. There is little good research to show that any one formulation is superior to another. However, patient preference is important since if happy with the form of NRT the more likely they are to comply with the regimen and complete the required course of treatment. It has been shown in a number of studies that many smokers using NRT in a quitting attempt fail too use sufficient NRT to suppress withdrawal symptoms and in this case are likely to blame the NRT product if the quitting attempt fails. Nicotine blood levels from NRT formulations (maximum daily dose) are generally 30% of the levels seen in smokers who smoke 20 cigarettes per day. The problem of overdose with NRT products is widely exaggerated. (Figure 2) Gum comes in two strengths 2mg and 4 mg per piece. Nicotine is released from the gum usually over 30 minutes of intermittent chewing and is mainly absorbed through the buccal mucosa. The recommended dosage is 8 to 12 pieces of the 2mg gum with a maximum daily dose of 15 pieces of the 4 mg gum. Proper instructions on use of the gum must be given so that smokers can benefit and avoid adverse effects. It’s important that clients keep to the chewing cycle. Users should chew the gum until the taste becomes strong, then the gum is parked between the gum and the teeth, when the taste fades the gum is taken back into the mouth and chewed until the taste becomes strong. This cycle is repeated over a 20 to 30 minute period for one piece of gum. Normally if a person smokes more than 20 cigarettes per day and smokes within 20 minutes of rising, they should start with the 4 mg gum. This is called the twenty-twenty rule and is used to determine the starting patch dose also – if the smokers fits the twenty-twenty rule they use the highest formulation. Patches Patches provide a steady state blood level of nicotine and do not mimic the peaks and troughs provided by other formulations. They are normally used for 10 to 12 weeks and come in three dosage levels which, over the weeks reduce the blood nicotine levels. Patches are divided into those that are use for 16 hours (taken off before sleep) and those that are 24 hours. Once removed the nicotine blood level falls off rapidly and therefore the use of the16 hour patch avoids sleep disturbance. Nicotine nasal spray consists of 10mg/ml of nicotine solution with a metered dosage delivery device. A single spray to the nostril delivers 0.5mg of nicotine. This is rapidly absorbed mostly through the lining of the nose. Peak plasma concentrations of nicotine are reached within 10 - 15 minutes. (Nicotine from a cigarette will reach these levels seconds after inhalation). The manufactures recommends one spray in each nostril, as required, up to a maximum of twice hourly for 16 hours in every 24 hours. It is targeted at, and there is some evidence that it has an effect, those patients who are highly dependent on nicotine – 40 to 60 cigarettes per day. Inhalator The inhalator involves a replaceable nicotine cartridge that is placed in a holder with mouth piece. Sucking on the inhalator allows nicotine vapour to be drawn into the mouth where it is absorbed into the blood via the buccal mucosa. No nicotine reaches the lungs. Each cartridge is used for a 20 minute session. Manufacturers advise that 6 to 12 cartridges are used daily for the first 8 weeks then reducing to zero in the next 4 weeks. Sublingual tablets The sublingual tablet is held under the tongue and allowed to dissolve over a thirty- minute period, during which it releases 2 mg of nicotine, 1 mg of which is absorbed. through the buccal mucosa. Manufacturers recommend 1-2 tablets hourly, depending on usual cigarette consumption, up to to a maximum of 40 tablets per day. Lozenge Is Nicotine Dangerous?Nicotine is only one element of tobacco smoke. The “tar” produced by smoking contains at least 4,000 different chemicals, including 50 known carcinogens. Other disease-causing elements include carbon monoxide, oxides of nitrogen and hydrogen cyanide. In essence nicotine, on it own, is as dangerous as caffeine and therefore presents little danger to public health. Inhaling tobacco smoke is a highly efficient method of absorbing nicotine into the blood stream. The amount absorbed from each cigarette is typically 1-2mg and it produces a rapid “hit” with the arterial peak nicotine levels 40-100ng/ml at about 15-30 seconds and a venous peak at about 5 minutes. These sharp peaks and troughs tend to rise over the first 6 –8 hours of the day with regular smoking and nicotine blood levels are also detected first thing in the morning (before the first smoke of the day) in regular smokers. It is the arterial peaks that are linked to the positive reinforcement of nicotine as an addictive drug. |
Days 1 – 3: |
0.5 mg once daily |
Days 4 – 7: |
0.5 mg twice daily |
Day 8 – End of treatment: |
1 mg twice daily |
The patient should set a date to stop smoking. Varenicline dosing should start 1-2 weeks before this date.
Patients should be treated with varenicline for 12 weeks. For patients who have successfully stopped smoking at the end of 12 weeks, an additional course of 12 weeks treatment with varenicline at 1 mg twice daily is allowed and there is some evidence that it increases the likelihood of long-term abstinence.
The main adverse effects associated with use of the drug were nausea sleep disturbances.
The efficacy of varenicline in smoking cessation was demonstrated in 3 clinical trials involving chronic cigarette smokers (³10 cigarettes per day).
Twoidentical double-blind clinical trials prospectively compared the efficacy of varenicline (1 mg twice daily), sustained release bupropion (150 mg twice daily) and placebo in smoking cessation. In these 52-week duration studies, patients received treatment for 12 weeks, followed by a 40-week non-treatment phase.
The primary endpoint of the two studies was the carbon monoxide (CO) confirmed, 4-week continuous quit rate from week 9 to week 12. The primary endpoint for varenicline demonstrated statistical superiority to bupropion and placebo with quit rates of 44.4% for varenicline, 29.5% for buproprion and 17.7% for placebo. These rate fell of to 22.1%, 16.4% and 8.4% respectively at 52 weeks.
Some caution is required in interpreting these results as the high success rate in the placebo group indicates a high level of support for smoking in the study and this is unlikely to be replicated in normal practice.
Conclusion
NRT is safe and effective and should be used by smokers wishing to quit. The recommendations issued by CSM and adopted by MHRA will go some way to reducing one of the barriers that smokers had with NRT particularly that it presents a risk to health. Pharmacists and their support staff can contribute to getting this message across not only to those wishing to stop but to all smokers as now there are more ways to use NRT in reducing the impact of smoking on health. But equally pharmacies should provide smoking cessation services that addresses the behavioural change needed to stop smoking.